Mylan Initiates Voluntary Nationwide Recall of Three Lots of Nizatidine Capsules, USP, Due to the Detection of Trace Amounts of NDMA (N-Nitrosodimethylamine) Impurity Found in the Active Pharmaceutical Ingredient Manufactured by Solara Active Pharma Scien
NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products and vegetables. NDMA has been classified as a probable human carcinogen (a substance that could cause cancer) according to the
The finished products are manufactured by
|
NDC |
Product Description |
Strength |
Size |
Lot Number |
Expiry |
|
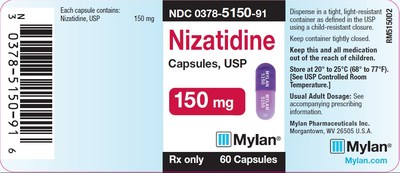
0378-5150-91 |
Nizatidine Capsules, USP |
150mg |
Bottles of 60 |
3086746 |
May 2020 |
|
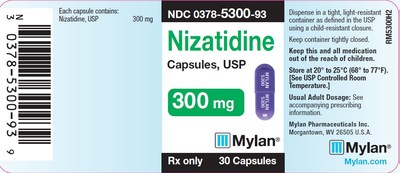
0378-5300-93 |
Nizatidine Capsules, USP |
300mg |
Bottles of 30 |
3082876 |
Jan 2020 |
|
0378-5300-93 |
Nizatidine Capsules, USP |
300mg |
Bottles of 30 |
3082877 |
Jan 2020 |
Nizatidine is indicated for the short-term treatment (up to 8 weeks) of active duodenal ulcers and active benign gastric ulcers, as maintenance therapy for duodenal ulcer patients for up to one year, and for up to 12 weeks for the treatment of endoscopically diagnosed esophagitis and associated heartburn due to gastroesophageal reflux disease (GERD).
Mylan is notifying its distributors and customers by letter and is arranging for return of all recalled products. Wholesalers, retailers and consumers that are in possession of recalled product should contact
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
- Adverse reactions or quality problems experienced with the use of this product may be reported to the
FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax. Complete and submit the report Online: www.fda.gov/medwatch/report.htm - Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-
FDA -0178.
This recall is being conducted with the knowledge of the
About Mylan
Mylan is a global pharmaceutical company committed to setting new standards in healthcare. Working together around the world to provide 7 billion people access to high quality medicine, we innovate to satisfy unmet needs; make reliability and service excellence a habit; do what's right, not what's easy; and impact the future through passionate global leadership. We offer a growing portfolio of more than 7,500 marketed products around the world, including antiretroviral therapies on which approximately 40% of people being treated for HIV/AIDS globally depend. We market our products in more than 165 countries and territories. We are one of the world's largest producers of active pharmaceutical ingredients. Every member of our approximately 35,000-strong workforce is dedicated to creating better health for a better world, one person at a time. Learn more at Mylan.com. We routinely post information that may be important to investors on our website at investor.mylan.com.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/mylan-initiates-voluntary-nationwide-recall-of-three-lots-of-nizatidine-capsules-usp-due-to-the-detection-of-trace-amounts-of-ndma-n-nitrosodimethylamine-impurity-found-in-the-active-pharmaceutical-ingredient-manufactured-by-s-300983578.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/mylan-initiates-voluntary-nationwide-recall-of-three-lots-of-nizatidine-capsules-usp-due-to-the-detection-of-trace-amounts-of-ndma-n-nitrosodimethylamine-impurity-found-in-the-active-pharmaceutical-ingredient-manufactured-by-s-300983578.html
SOURCE
Christine Waller (Media), 724.514.1968; Melissa Trombetta (Investors), 724.514.1813
